The cellular diversity of the human brain is immense, which is generated through evolutionarily conserved developmental mechanisms that are well-timed. The system is robust enough to create functional masterpiece; yet is susceptible to failure that may lead to cognitive dysfunction or malignant growth.
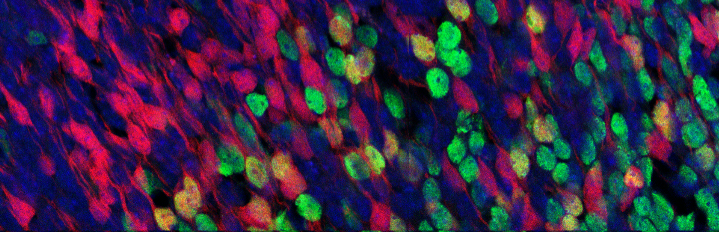
We study the molecular and cellular mechanisms underlying the functional diversity of the adult reward system, cell fate choices leading to its development and how these are linked to the progression of time. For this, we use state-of-the art single cell RNA/epigenome sequencing techniques, CRISPR-mediated gene inactivation, mouse genetics, bioengineered organoids and computational tools to run multidisciplinary, collaborative projects.
Latest news- The Shapeshifters Symposium; Plasticity – Here, There, and Everywhere
Join and contribute to the The Shapeshifters Symposium; Plasticity – Here, There, and Everywhere!
Plasticity, the ability to be molded in various forms while maintaining a core identity, is a term that is increasingly used within various fields of science, e.g. neuroscience, plant- and cell biology, and within the humanities. However, the meaning and use of plasticity varies between these fields. How are these different usages – from shapeshifting to adaptability, related between disciplines, and how can plasticity be developed into a threshold concept within fields where it is currently not in use?
The Shapeshifters Symposium is a transdisciplinary two-day event that explores the concept of plasticity across academic domains and beyond. We, a group of interdisciplinary scholars, invite researchers, societal stakeholders and artists to come together and question what it means to be a shape within a shapeshifting society, a form within a form – adapting, evolving and mutating, along with its environment. And you are warmly invited to join us!
This event is organised by the Plasticity team, a group of interdisciplinary researchers working on the concept of plasticity, sponsored by the Dutch Centre for Unusual Collaborations. Two panel facilitators will frame the topic of the panel with a statement from the perspective of their background. After that, the conversation will be opened to all people to present ‘fishbowl-style’ – meaning everybody is invited to join (and leave) the available seats on stage and add to the discussion with questions and thoughts.
“We hope to further expand the concept of plasticity across disciplines – and we need your help!” The Plasticity Team

Day One of the symposium, May 30th, consists of four panels that are transdisciplinary in nature:
09:00 Registration & Coffee
09:30 – 11:00 Panel I – Plasticity, Complexity, and Circular Causality
Moderator: Yaron Caspi
Panel facilitators: Prof. dr. Ray Noble & Prof. dr. Peter Sloot11:30 – 13:00 Panel II – Plasticity from within and from without
Moderators: Dr. Esmee Geerken & Dr. Yaron Caspi
Panel facilitators: Dr. Danqing Lui & Prof. dr. Frank Seebacher14:00 – 15:30 Panel III – Time & Mind
Moderators: Tamalone van den Eijnden & Dr. Onur Basak
Panel facilitators: Dr. Kjetel Horn Hogstad & Dr. Joost de Jong16:00 – 17:30 Panel IV – Meaning making across epistemic cultures
Moderators: Dr. Jeff Diamanti & Dr. Abby Waysdorf
Panel facilitators: Alice Iacobone & Prof. Dr. Amanda BoetzkesPanel descriptions and the programme can be found on our event page. Our PIs Onur Basak, PhD (leading the consortium) and prof. Elly Hol, PhD are members of this team. The translational Neuroscience department is a hub for brain plasticity research.
- PhD student position in molecular and cellular neurophysiology [CLOSED]
The groups of Frank Meye and Onur Basak have a PhD student position in molecular and cellular neurophysiology, in the context of the BRAINSCAPES consortium. For 4 years, the student will be neurophysiologically/transcriptomically identifying distinct mouse midbrain neurons, and virally targeting them. Terms of employment in accordance with the CAO hospitals and UMCU salary scales.

We seek an outstanding and motivated PhD candidate with an MSc in physiological and/or molecular neuroscience. Rodent work expertise is required. Experience with patch clamp physiology and/or molecular techniques are advantages. If you have a background in slice neurophysiology and/or molecular neuroscience and are interested in identifying which neurons in the brain are relevant to mental disorders, don’t miss this PhD student opportunity!
How to apply
You can apply through the UMC Utrecht website. See the post here with the details of the position. For additional information, please contact Dr. Frank Meye (fjmeye-2@umcutrecht.nl) and/or Dr. Onur Basak (O.Basak@umcutrecht.nl).
This is where you will work

The Department of Translational Neuroscience is the preclinical department of the Division Neuroscience of the University Medical Center Utrecht, forming the cor of fundamental research at the Center for translational Neuroscience. The research mission of the Department of Translational Neuroscience is to discover and delineate mechanisms and processes which are fundamental to the development of neural systems and to the control of behavior, and to translate these to pathogenesis and disease models.
The unique advantage of this department of Neuroscience is its embedding in the clinical environment of UMCU and its multidisciplinary character. The Department’s toolkit includes light sheet microscopy, optogenetics, electrophysiology, an own animal facility, viral vector generation and more.
You take this with you
As a PhD student, you will work on your own project, supported by technicians and surrounded by a team of PhD students and postdocs that work with similar and other technologies. The project focuses on disentangling functionally distinct midbrain neurons with roles in coding stress or reward (cues), and/or with specific projection targets. These aspects are important in drug addiction, eating disorders and depression. It is a full-time position, which will lead to a PhD thesis. Knowledge of neuroscience and experience with rodent handling are required. Familiarity with slice electrophysiology (eg patch clamp) and/or molecular or transcriptomic techniques are strengths. The candidate needs to have good writing and presenting skills (in English).
We seek a highly motivated and experienced PhD candidate with strong expertise in molecular and/or cellular physiological neurobiology. It is essential that the applicant is able and interested in workingin a unique multidisciplinary team with researchers from several universities involved in this project. Knowledge of neuroscience and experience with rodent handling are required. Familiarity with slice electrophysiology (eg patch clamp) and/or molecular, tr
anscriptomic or bioinformatics techniques are strengths. The candidate needs to be proficient in English.
For additional information, please contact Dr. Frank Meye ( fjmeye-2@umcutrecht.nl ) and/or Dr. Onur Basak ( O.Basak@umcutrecht.nl ).
This is what we offer you
- A salary between €2901 and €3677 gross per month (salary scale OIOs), based on full-time employment (36 hours).
- Year-end bonus of 8.3% and holiday allowance of 8%.
- Pension insurance with ABP. We take care of approximately 70% of the monthly contribution.
- Access to a variety of online courses through GoodHabitz.
- The option to select additional employment benefits in exchange for gross salary, such as purchasing a bicycle and memberships.


